Significant Achievement Cements the Company's Leadership in Cardiac Data Management SARASOTA, Fla.--(BUSINESS WIRE)--PaceMate®, a market-leading...


Significant Achievement Cements the Company's Leadership in Cardiac Data Management SARASOTA, Fla.--(BUSINESS WIRE)--PaceMate®, a market-leading...

NEW YORK, March 11, 2024 /PRNewswire/ -- UpLift, a technology-driven behavioral health company delivering in-network therapy and psychiatry, today...

Work Shield will use the proceeds of the growth capital to invest across all functional areas of the business. DALLAS, Nov. 1,...

Established leader adds financial technology and services expertise to executive team. BIRMINGHAM, AL. – September 6, 2023 – Dash Solutions,...

SARASOTA, Fla.--(BUSINESS WIRE)--PaceMate®, a market leading cardiac data management platform, announced today a strategic growth investment...

UpLift is contracting with AmeriHealth Caritas to form a first-of-its-kind clinically integrated network to expand behavioral healthcare access for...

Intelligent Retinal Imaging Systems (IRIS) is a Pensacola, Fla.-based company founded in 2011 by nationally recognized retina surgeon Dr. Sunil...

Leadership transition positions YPrime for next phase of growth while ensuring continuity of the company's mission to deliver innovative and...

US: sojo, which describes itself as the vacation rental industry’s first automated amenity platform, has raised $6.2 million in a Series A funding...
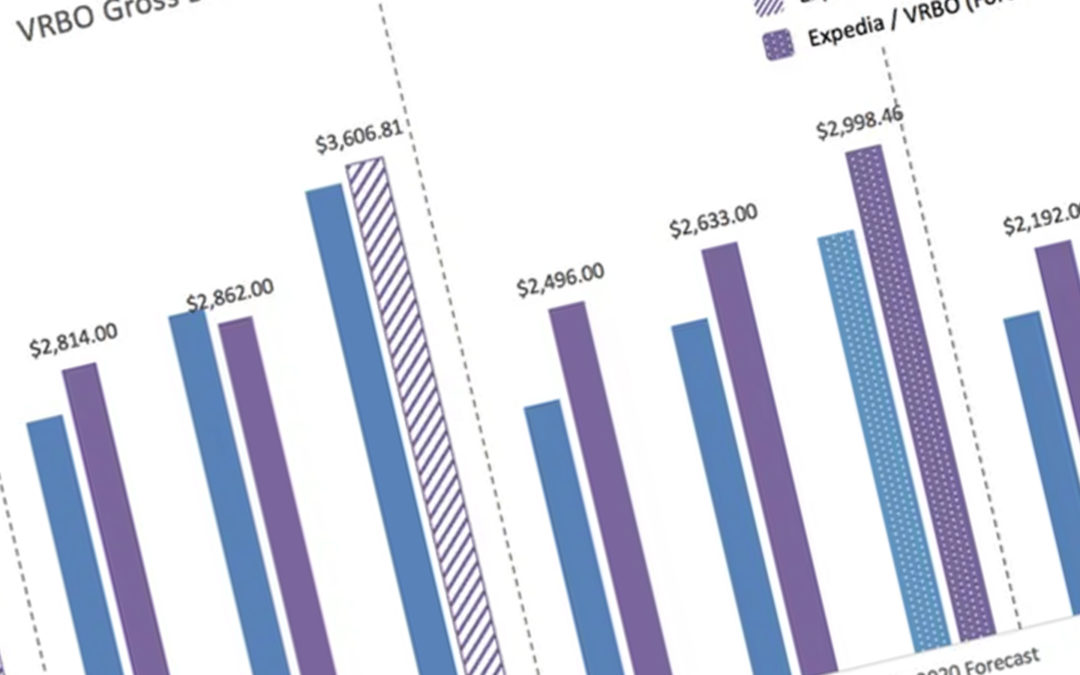
The combination of forward-looking market insights will produce the industry’s most accurate view of worldwide air, hotel, and alternative...